

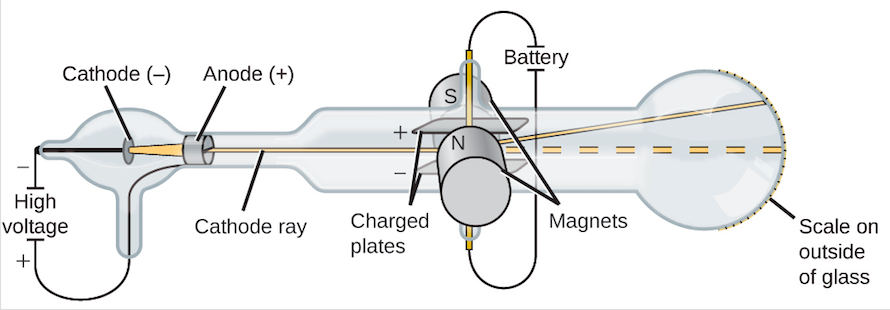
When deflected by the magnetic field, it moves in the arc of a circle.When the particle is deflected by the electric field, it experiences a force F=qE.A fluorescent screen showed where the particle hit. He placed electric and magnetic fields at right angles to each other so that their forces would completely negate each other. Thomson used a modified version of a cathode ray tube.Thomson performed an experiment using cathode rays to determine the ratio of the charge of cathode rays to their mass.Experimental aim: Thomson, through this experiment, calculated the charge to mass ratio ( q/m) of cathode rays.It also showed that the ray was produced from the cathode.This supported the particle side of the controversy as electromagnetic waves are massless. When the glass wheel was struck by the cathode ray, the glass wheel slowly rotated, indicating that the cathode rays have momentum and therefore mass.

Observations from the experiments confused some scientists as the rays showed properties of both the wave and particles.After Cathode Ray was discovered in 1855, many experiments were done with them in order to understand their nature.
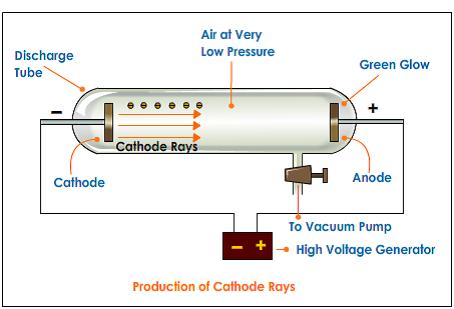
However, at reduced pressure, air becomes conductive.Īre cathode rays charged particles or electromagnetic waves? Under normal conditions of temperature and pressure, air acts as an insulator.The high voltage is essential to overcome the poor conductivity of a near vacuum environment (most likely gas).When a high voltage is connected between the electrodes, an invisible ray (cathode ray) travels from the cathode to the anode. The positive electrode is the anode, and the negative electrode is the cathode.By the end of the 19 th Century, vacuum pumps were perfected, and glass tubes were being made.Investigation of vacuum tubes could not occur until good vacuum pumps had been invented.Cathode rays were produced in cathode ray tubes which are vacuum tube is a discharge tube consisting of two electrodes enclosed within a sealed glass or metallic chamber, from which the air has been withdrawn to create a near-vacuum environment.– Millikan's oil drop experiment (ACSPH026) What are cathode rays? – early experiments examining the nature of cathode rays investigate, assess and model the experimental evidence supporting the existence and properties of the electron, including:.


 0 kommentar(er)
0 kommentar(er)
